EN ISO 17664 pdf download

EN ISO 17664 pdf download Sterilization of medical devices – Information to be provided by the manufacturer for the processing of resterilizable medical devices
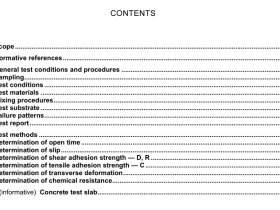
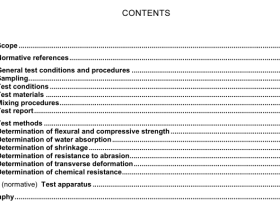
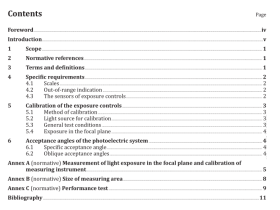
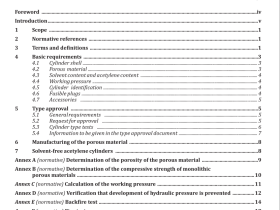
1 Scope
This standard specifies the information to be provided by the medical device manufacturer on the processing of medical devices claimed to be re-sterilizable and medical devices intended to be sterilized by the processor.
This standard specifies requirements for the information to be provided by the medical device manufacturer, so that the medical device can be processed safely and will continue to meet its performance specification.
Requirements are specified for processing that consists of all or some of the following activities:
preparation at the point of use;
preparation, cleaning, disinfection;
drying;
inspection, maintenance and testing;
packaging;
sterilization;
storage.
When providing instructions for these activities, medical device manufacturers are expected to be aware of the training and knowledge of procedures, and of the processing equipment available to the persons likely to be responsible for processing. It is likely that some processing procedures will be generic and well known and will use equipment and consumables conforming to recognized standards. In this case, a reference in the instructions is all that is required. For those medical devices where instructions for use are not required to accompany the medical device, other means of communicating the information can be used, e.g. user manuals, symbols or wall charts supplied separately.
This standard excludes textile devices used in patient draping systems or surgical clothing.
NOTE The principles of this standard may be applied when considering the information to be supplied with medical devices which only require disinfection prior to re-use.
2 Terms and definitions
For the purposes of this European Standard, the following terms and definitions apply.
2.1
chemical
formulation of compounds intended for use in reprocessing
NOTE This includes, for example, detergents, surfactants, rinse aids, disinfectants, enzymatic cleaners,sterilants.
2.2
cleaning
removal of contamination from an item to the extent necessary for further processing or for intended use 2.3 disinfection process used to reduce the number of viable microorganisms on a product to a level previously specified as appropriate for its further handling or use
2.4
manual cleaning
cleaning without the use of a washer-disinfector
2.5
manufacturer
organization with responsibility for the design, manufacture, packaging and labelling of a device before it is placed on the market under its own name, regardless of whether these operations are carried out by that person himself or on its behalf by a third party