EN ISO 11607-2 pdf download

EN ISO 11607-2 pdf download Packaging for terminally sterilized medical devices – Part 2: Validation requirements for forming, sealing and assembly processes (ISO 11607-2:2006)
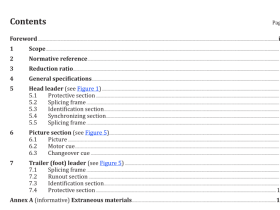
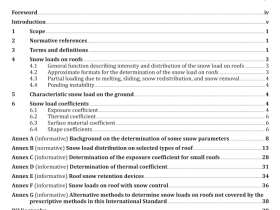
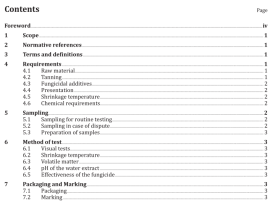
Scope
This part of ISO 11607 specifies the requirements for development and validation of processes for packagingmedical devices that are terminally sterilized. These processes include forming, sealing, and assembly otpreformed sterile barrier systems, sterile barrier systems and packaging systems.
This part of ISO 11607 is applicable to industry, to health care facilities, and wherever medical devices arepackaged and sterilized
This part of ISO 11607 does not cover all requirements for packaging medical devices that are manufacturedaseptically. Additional requirements may also be necessary for drug/device combinations.
2 Normative references
The following referenced documents are indispensable for the application of this document. For datedreferences. only the edition cited applies. For undated references, the latest edition of the referenceddocument (including any amendments) applies.
ISO 11607-1, Packaging for terminally sterilized medical devices – Part 1: Requirements for materials, sterilebarrier systems and packaging systems
3 Terms and definitions
For the purposes of this document, the following terms and definitions apply.
3,1
expiry date
indication of the date, by which the product should be used, expressed at least as the year and month
3.2
installation qualification9
process of obtaining and documenting evidence that equipment has been provided and installed inaccordance with its specification
ISO/TS 11139:2006
3.3
labelling
written, printed, electronic or graphic matter affixed to a medical device or its packaging system; or accompanying a medical device
NOTE Labelling is related to identification, technical description and use of the medical device but excludes shipping documents.
3.4
operational qualification
OQ
process of obtaining and documenting evidence that installed equipment operates within predetermined limits
when used in accordance with its operational procedures
[ISO/TS 11139:2006]
3.5
packaging system
combination of the sterile barrier system and protective packaging
[ISO/TS 11139:2006]
3.6
performance qualification
PQ
process of obtaining and documenting evidence that the equipment, as installed and operated in accordance with operational procedures, consistently performs in accordance with predetermined criteria and thereby yields product meeting its specification
[ISO/TS 11139:2006]
3.7
preformed sterile barrier system
sterile barrier system that is supplied partially assembled for filling and final closure or sealing EXAMPLE Pouches, bags and open reusable containers
[ISO/TS 11139:2006]
3.8
process development
establishing the nominal values and limit(s) for critical process parameters
3.9
product
result of a process
[ISO 9000:2000]
NOTE For the purpose of sterilization standards, product is tangible and can be raw material(s), intermediate(s), subassembly(ies) and health care product(s).
[ISO/TS 11139:2006]
3.10
protective packaging
configuration of materials designed to prevent damage to the sterile barrier system and its contents until the point of use
[ISO/TS 11139:2006]